Molnupiravir
Antiviral medication
Molnupiravir ▸ Facts ▸ Comments ▸ News ▸ Videos
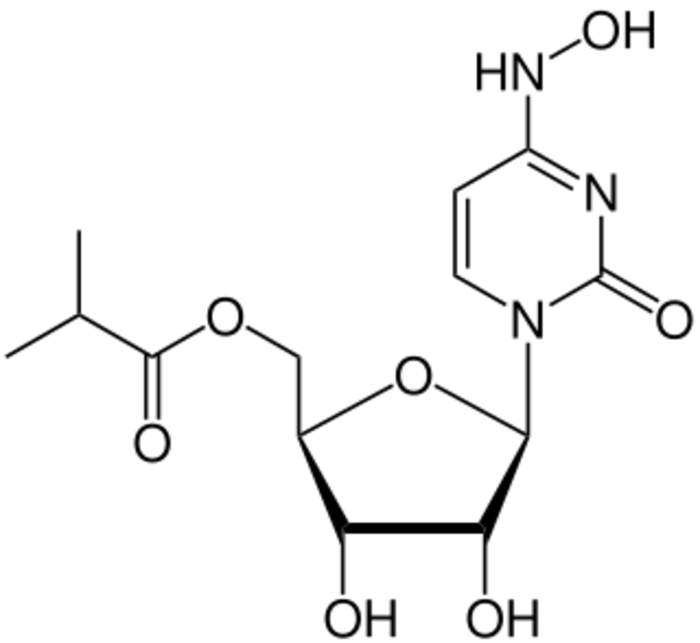
Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2. It is taken by mouth.
| 0 shares | ShareTweetSavePostSend |
You Might Like
Amid Covid surge, ICMR cautions on antibiotic useThe Indian Council of Medical Research has issued revised guidelines for Covid-19 in the wake of the surge of cases in the past week across the country. “The guideline does not advice the use of..IndiaTimes - Published |
| Search this site and the web: |
