Booster Shots in Children Ages 5–11 Increase Omicron Antibodies, Pfizer Says
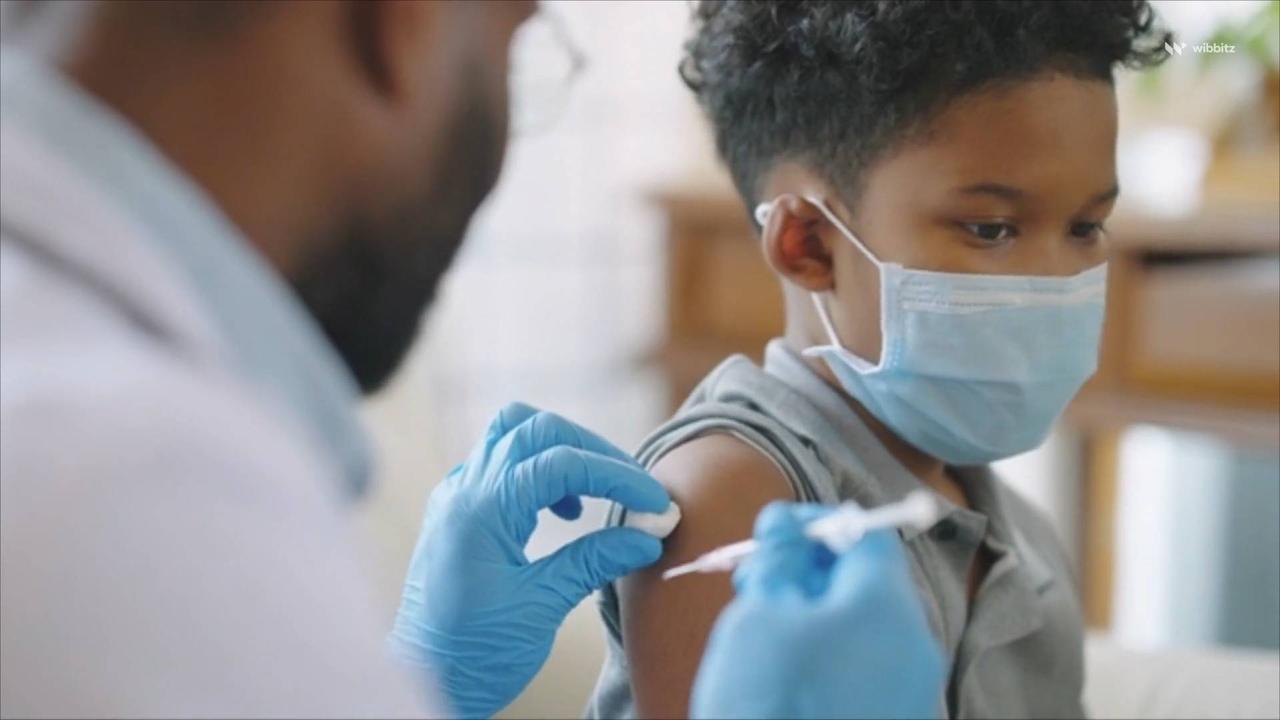
Booster Shots in Children Ages 5–11 Increase Omicron Antibodies, Pfizer Says
Booster Shots in Children Ages 5–11 , Increase Omicron Antibodies, Pfizer Says.
NBC News reports a booster shot of the Pfizer-BioNTech coronavirus vaccine increases antibody protection in children ages 5-11, the company said on April 14.
NBC News reports a booster shot of the Pfizer-BioNTech coronavirus vaccine increases antibody protection in children ages 5-11, the company said on April 14.
Given half a year after the initial series of two doses, a booster shot of the Pfizer-BioNTech vaccine increased antibody levels sixfold.
In Pfizer's clinical trial, 140 children ages 5 through 11 received a booster shot.
According to NBC News, data has not yet been made available to independent researchers for review.
In the era of Omicron, booster doses of COVID vaccines have been hit and miss.
In adults, a booster dose of Pfizer's vaccine wanes dramatically after four months.
Health experts say the same trends are likely to occur in children.
For adults, Pfizer's vaccine is 30 micrograms per dose.
For children, vaccines are 10 micrograms per dose.
Earlier this year, New York Department of Health researchers found Pfizer's vaccine to offer minimal protection against infection in children.
But according to NBC, the CDC later said two doses in children offered ample protection against severe infections.
But according to NBC, the CDC later said two doses in children offered ample protection against severe infections



![Trader Joe’s Recalls Basil After Reports of Salmonella Infections [Video]](https://video.newsserve.net/300/v/20240418/1373034356-Trader-Joe-Recalls-Basil-After-Reports-of.jpg)
![Today Is World Autism Awareness Day [Video]](https://video.newsserve.net/300/v/20240402/1371588730-Today-Is-World-Autism-Awareness-Day.jpg)
![US Life Expectancy on the Rise Following Pandemic Decline, CDC Report Says [Video]](https://video.newsserve.net/300/v/20240328/1371197512-US-Life-Expectancy-on-the-Rise-Following-Pandemic.jpg)
![3 Out of 4 Kids in the US Have Had COVID, CDC Estimates [Video]](https://video.newsserve.net/300/v/20220427/1334989468-Out-of-Kids-in-the-US.jpg)
![Moderna Working on Vaccine Booster That Targets the Omicron Variant [Video]](https://video.newsserve.net/300/v/20220419/1334614051-Moderna-Working-on-Vaccine-Booster-That-Targets-the.jpg)
![What's Next for COVID-19 Vaccines? [Video]](https://video.newsserve.net/300/v/20220405/1333898331-What-Next-for-COVID-19-Vaccines.jpg)