ZyCoV-D
Vaccine candidate against COVID-19
ZyCoV-D ▸ Facts ▸ Comments ▸ News ▸ Videos
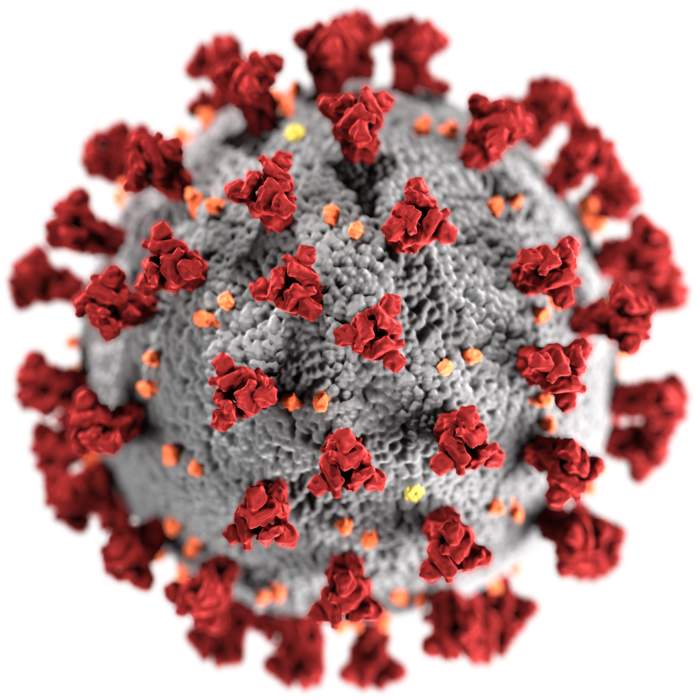
ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.
| 0 shares | ShareTweetSavePostSend |
You Might Like
No news matches foundSorry, we were unable to find any results in our database for your queryFree news archive accessDid you know? You are eligible to search our news archive with millions of news references free of charge. To do this, please sign in first at the top of the screen. • Information about free access to our news archive Search this site and the web:  |
