Moderna COVID-19 vaccine
RNA COVID-19 vaccine
Moderna COVID-19 vaccine ▸ Facts ▸ Comments ▸ News ▸ Videos
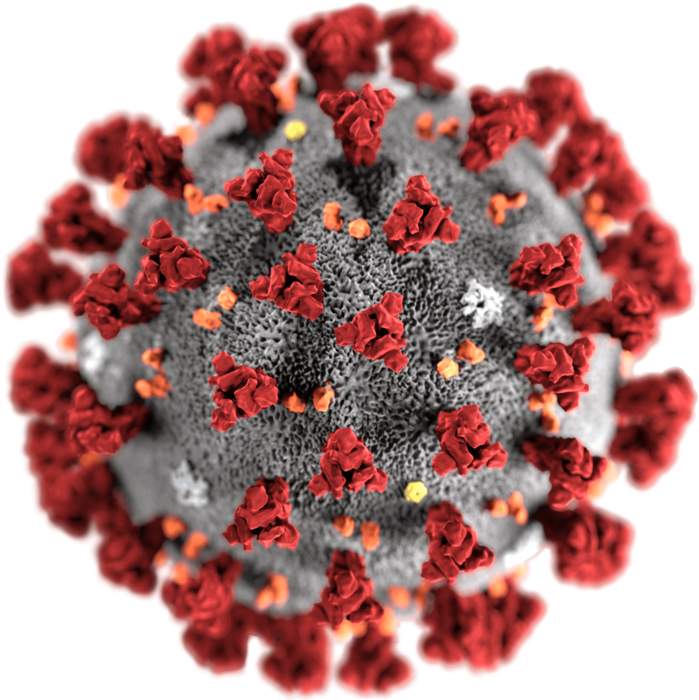
The Moderna COVID‑19 vaccine, sold under the brand name Spikevax, is a COVID-19 vaccine developed by the American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in humans aged six months, twelve years, or eighteen years and older. It provides protection against COVID-19, which is caused by infection by the SARS-CoV-2 virus.
| 1 shares | ShareTweetSavePostSend |
You Might Like
No news matches foundSorry, we were unable to find any results in our database for your queryFree news archive accessDid you know? You are eligible to search our news archive with millions of news references free of charge. To do this, please sign in first at the top of the screen. • Information about free access to our news archive Search this site and the web:  |
